Background:
Idiopathic multicentric Castleman disease (iMCD) is a rare, atypical hematologic disorder characterized by diffuse lymphadenopathy and systemic inflammation causing potentially fatal multi-organ system dysfunction. Though the etiology remains unknown, interleukin-6 (IL-6) has been implicated in disease pathogenesis and siltuximab, a monoclonal antibody that antagonizes IL-6, is the only FDA-approved iMCD treatment. Despite substantial morbidity for children with iMCD, the pediatric experience has not been well characterized. It is unclear whether insights gleaned from adults apply to children or whether siltuximab, which was never prospectively studied in a pediatric population, achieves similar response rates in children. We sought to correct this knowledge gap and better characterize pediatric iMCD.
Methods:
The ACCELERATE longitudinal Castleman disease (CD) natural history registry was interrogated for patients under 18 years of age at time of diagnosis. For each patient, complete medical history and lymph node biopsy slides were collected and extracted, and a panel of clinicians and pathologists performed independent adjudication of the diagnosis. Demographics - including age at diagnosis, histopathologic subtype, clinical subtype, clinical symptoms, and most severe laboratory parameters present within ± 90 days from date of pathological diagnosis were aggregated. Lastly, treatments administered to the cohort of pediatric patients were inventoried and both clinical response (defined as at least 50% reduction in abnormal clinical and laboratory criteria) and durable response (defined as a positive clinical response lasting ≥ 365 days with no other CD treating drug administered in between) were determined.
Results:
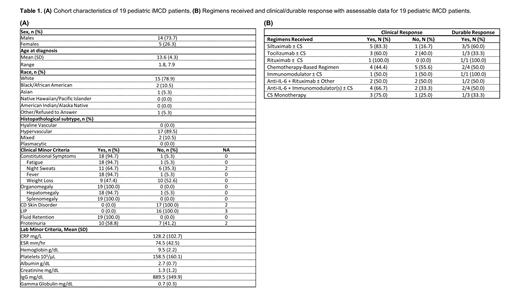
Nineteen pediatric patients were confirmed to have iMCD, of which 14 (74%) were male and 5 (26%) were female (Table 1A). The mean (standard deviation [SD]) age at diagnosis was 13.6 (4.3), with the youngest patient diagnosed at 2 years of age. Much of the cohort demonstrated hypervascular lymph node histology (89.5%). Clinically, 84% of the patients demonstrated the severe TAFRO (thrombocytopenia, anasarca, fever, renal dysfunction/reticulin fibrosis, organomegaly) subtype while 16% were not otherwise specified. 95% of patients presented with constitutional symptoms and all but one patient presented with fatigue and fevers. All 19 patients presented with organomegaly and fluid retention (anasarca, ascites, pleural effusions, and/or peripheral edema). No patients demonstrated CD-specific skin abnormalities (violaceous lymphocytic papules or cherry hemangiomata) or interstitial lymphocytic pneumonitis. Labs indicated significant systemic inflammation, with mean (SD) C-reactive protein, erythrocyte sedimentation rates, and albumin values of 128.3 (102.7) mg/L, 74.5 (42.5) mm/hr, and 2.7 (0.6) g/dL, respectively. Eighteen (95%) patients presented with anemia, with a mean (SD) hemoglobin value of 9.5 (2.2) g/dL. Sixteen (84%) patients demonstrated platelet abnormalities, with 13 patients presenting with thrombocytopenia and three with thrombocytosis. No patient demonstrated hypergammaglobulinemia. Nine patients received three unique siltuximab regimens: siltuximab ± CS (corticosteroids), siltuximab + sirolimus ± CS, and siltuximab + other (other being defined as all other treatments such as rituximab, eculizumab, and ravulizumab) (Table 1B). Siltuximab ± CS was administered to six patients; 83% (n=5) had a positive response, and 60% of responders achieved a durable response. Lastly, two patients with TAFRO died from their disease.
Conclusions:
Overall, real-world medical record data from a cohort of pediatric iMCD patients enrolled in ACCELERATE reveals high disease burden within this population. A notable degree of TAFRO, the most severe iMCD subtype, was observed within this pediatric cohort, with clinical manifestations and laboratory abnormalities commonly observed in adult iMCD. This makes this population particularly vulnerable and highlights the need for prompt and accurate treatment. We also observed a high response rate to siltuximab ± CS in this small cohort, supporting its use in pediatric iMCD. Further research examining the effectiveness of siltuximab in a larger cohort of pediatric patients as well as other treatments is needed to identify optimal treatment approaches.
OffLabel Disclosure:
Brandstadter:EUSA Pharma: Consultancy. van Rhee:Bristol Myers Squibb: Research Funding; Adicet Bio: Consultancy; GlaxoSmithKline: Consultancy; Janssen Pharmaceuticals: Research Funding; EUSA Bio: Consultancy. Fajgenbaum:sobi: Consultancy; EUSA Pharma LLC (US), which has merged with Recordati Rare Diseases Inc. (2018 to 2023): Consultancy, Research Funding; Pfizer: Other: Study drug for clinical trial of sirolimus; N/A: Other: Holds pending provisional patents for ‘Methods of treating idiopathic multicentric Castleman disease with JAK1/2 inhibition’ and ‘Discovery and validation of a novel subgroup and therapeutic target in idiopathic multicentric Castleman disease’.
This abstract evaluates treatment outcomes of tocilizumab, rituximab, corticosteroids, cytotoxic chemotherapy, and/or complement inhibitors in pediatric patients with idiopathic multicentric Castleman disease (iMCD). These are off-label drugs and are recommended for patients when siltuximab, the only FDA-approved drug for iMCD, is not available or is not effective in inducing a clinical response.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal